
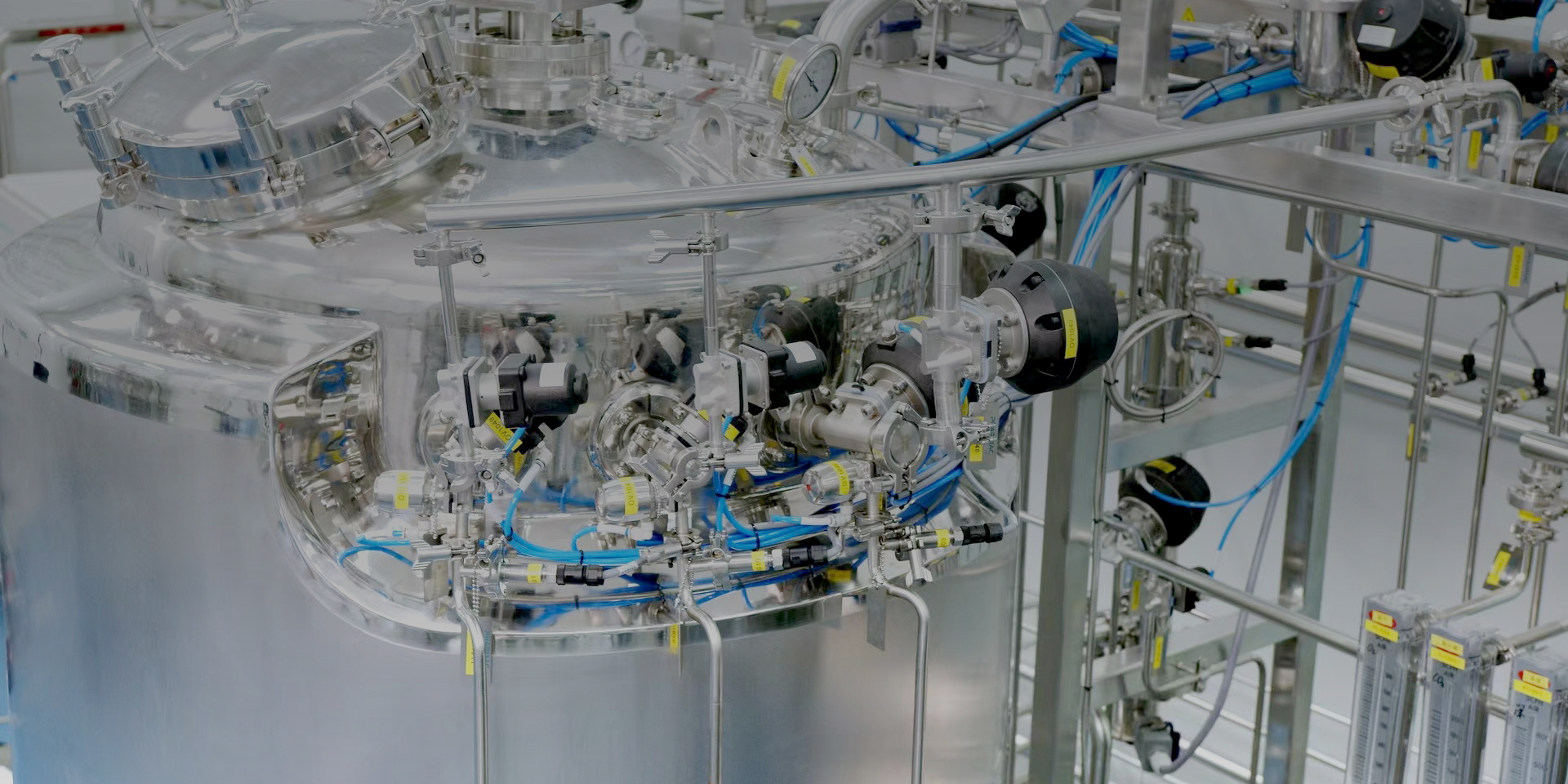
Application of large-scale DCS system in synthetic biology production
Currently, the biosynthesis manufacturing industry faces several challenges, including incomplete core technologies, low levels of industrial maturity, and unclear commercialization paths. These challenges have significantly delayed the promised arrival of a "green new future." In response to these challenges, a leading global biotechnology company has established an end-to-end pilot platform, bridging the gap from biological design to scale-up and industrial production. They have chosen Pharmac as the supplier of core process equipment and automation systems.

As a global leader in biosynthetic product pilot platforms, it is crucial to address the flexible production needs of different clients and products. For this project, Pharmac selected the Siemens PCS7 system as the production control system, tailored to the specific needs of the client. This system features dual redundancy for CPU, communication bus, power supply, and servers. Each subsystem is equipped with operator stations that can function independently or as backups, ensuring safety, reliability, and advanced technology that meets the stringent control requirements of biosynthetic product manufacturing. The system offers powerful editing capabilities for process parameters and workflow logic formulas, accommodating the diverse process requirements throughout the entire production line lifecycle.

Based on automation technology, core concepts of Industry 4.0 such as digital twins, IoT, and integration of IT and OT are essential. To harness the data potential of the automation system and enhance product quality and operational efficiency, the system provides an open OPC UA data interface, facilitating data sharing and integration among various digital factory systems. The project client is equipped with exhaust gas analysis equipment. The exhaust gas mass spectrometer measures data such as water, oxygen, nitrogen, argon, carbon dioxide, ammonia, methanol, and ethanol in the exhaust gases. During fermentation, changes in the concentration of exhaust gas components reflect changes in the fermentation process, particularly variations in carbon dioxide and oxygen, which indicate the fermentation state and microbial growth conditions. The fermentation equipment control system uses the OPC UA communication protocol to read exhaust gas analyzer data and calculates CER (carbon dioxide evolution rate), OUR (oxygen uptake rate), and RQ (respiratory quotient) based on other analysis parameters. This data allows for adjustments to stirring speed, feeding rate, and aeration volume, optimizing the fermentation process and improving fermentation quality and yield.
The exhaust gas analyzer can simultaneously collect data from one reference gas and 15 fermentation tanks, automatically switching for analysis, performing independent calculations, and transmitting data to a server computer for display and storage. Online monitoring of exhaust gases and parameter calculations allows for easy assessment of fermentation stages, provides valuable insights into microbial fermentation patterns, and guides the adjustment of aeration and stirring speed parameters.
The project has now entered formal production. Under 24×7 high-load operations, the system has proven very stable, effectively meeting the frequent product changes on the production line. The quality of the produced products has also exceeded previous standards, and fully automated operations have greatly enhanced production efficiency.
From design to implementation, Pharmac offers a one-stop solution for clean fluid process equipment in the pharmaceutical industry.