
Common Issues in the CIP Process
CIP Issues Have Always Been a Pain Point and Challenge in Large Biopharmaceutical Tank-Pipeline System Engineering.
To address this, the Pharmac team has initiated a series of discussions on common issues in the CIP (Clean-In-Place) process. Only through exploration can we accurately target the research of new technologies.


Recently, the Pharmac team received feedback from several customers who had purchased stainless steel media and buffer system. They reported that the CIP cleaning time of equipment provided by other suppliers was extremely long, the conductivity metrics never met the cleaning requirements, and some equipment even had WFI (Water for Injection) final rinse times exceeding one hour. We believe this is highly unreasonable, not only leading to wasted cleaning media but also potentially causing production scheduling risks, which could lead to more severe consequences. Therefore, the Pharmac team conducted an in-depth investigation into this feedback.
The investigation found that these issues were frequently concentrated in the same operating condition: one CIP station had to serve both large and small equipment or pipelines simultaneously, resulting in excessively long CIP times when cleaning small equipment or pipelines.
Part 1: Cause Analysis
The factors that influence CIP are mainly temperature, time, mechanical action, and chemical action. The relationship between these four factors and CIP cleaning effectiveness can be represented by the TACT model:
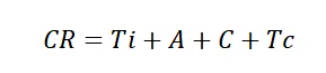
CR: Cleaning effectiveness;
Ti: Time;
A: Mechanical action;
C: Chemical action;
Tc: Temperature.
Image: Four Main Factors of CIP
The endpoint of CIP cleaning is usually determined by conductivity. When conductivity meets the required standards, if the cleaning effectiveness (CR) is consistent, and the chemical action (C, specifically the concentration of the cleaning agent) and temperature (Tc) of the same cleaning formula remain constant, then a prolonged cleaning time (Ti) indicates a deficiency in mechanical action (A).
In the CIP system, mechanical action primarily comes from the turbulence of the fluid. Turbulence can be calculated using the Reynolds number, which is proportional to flow velocity under constant conditions. The higher the flow velocity, the higher the Reynolds number, and the greater the turbulence. This results in shorter cleaning times under the same operating conditions. The relationship between the Reynolds number and flow velocity can be represented by the following equation:
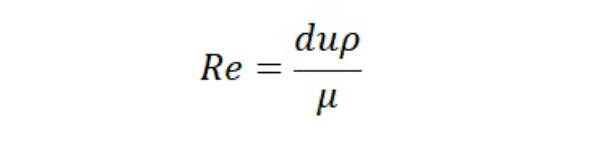
Re: Reynolds number
d: Pipe diameter, m
u: Flow velocity, m/s
ρ: Density, kg/m3
μ: Dynamic viscosity, Pa·s
However, excessive flow velocity can also lead to other adverse effects, such as increased pipeline losses and a higher risk of water hammer. According to the Fanning equation, the higher the flow velocity, the greater the friction loss along the pipeline:
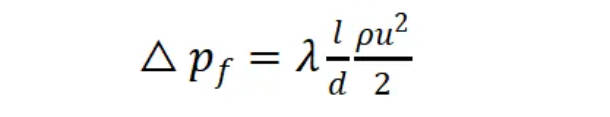
Δpf: Friction loss per unit volume of fluid, Pa
l: Pipeline length, m
d: Pipe diameter, m
u: Flow velocity, m/s
ρ: Density, kg/m3
λ: Friction coefficient
Excessive pipeline loss can result in larger pump selection, ultimately leading to increased initial equipment investment and long-term energy consumption. This issue can be entirely avoided during the early design stages.
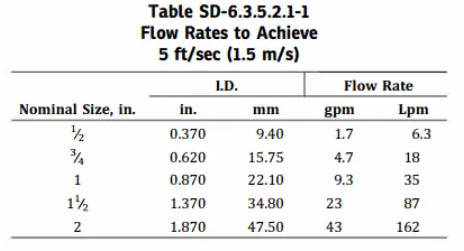
Image: ASME BPE Standard Recommendations
The ASME BPE standard recommends that CIP flow velocities should reach 1.5 m/s. However, this velocity is not fixed and should be considered comprehensively based on different pipeline layouts, media flow directions, pipe diameters, and related components.
The analysis shows that the main reason for these issues is that the flow rate required to clean small pipes or equipment is relatively low, leading to reduced flow velocities in the overall CIP circulation pipeline. This reduces the overall turbulence in the pipeline, lowers the mechanical scrubbing force, and increases the cleaning time under the same cleaning requirements and media conditions. The root cause of the problem often lies in the difficulty of cleaning the return water main rather than the target equipment or pipeline itself.
However, in large biopharmaceutical tank-pipeline systems, it is often unavoidable to use a single CIP station to clean equipment or pipelines of significantly different sizes due to process or overall cost considerations. As a result, CIP distribution pipelines, especially return water pipelines, need to be larger to match the cleaning requirements of the largest tank or equipment, leading to the issue of cleaning small pipes with large pipes. This not only increases cleaning time and wastes cleaning media but also shortens the pump's lifespan due to pressure buildup. For example, in a common affinity chromatography scenario:
The sample loading tank and sample collection tank for affinity chromatography sometimes differ in volume by a factor of ten. The sample loading pipelines for chromatography are often small, such as in a monoclonal antibody project: the sample loading tank for affinity chromatography has a volume of 8,000L, the sample collection tank only 800L, and the sample loading pipeline is only 3/4". However, for cleaning the sample loading tank, depending on the layout, the CIP supply main may need to reach 2", and the return main may be 2" or even 2.5".
Without special design, when cleaning the 3/4" pipeline, the total flow rate may only reach around 1400L/h, and the flow velocity in the 2" pipeline may only reach 0.2m/s, far below the 1.5m/s, with a Reynolds number of only around 10,000. Although this is within the turbulent range, the mechanical action is significantly reduced, inevitably increasing cleaning time.
The Pharmac team performs detailed calculations for each specific operating condition to avoid cleaning difficulties due to flow velocity issues and to prevent the time and opportunity cost wasted on later modifications.
During pipeline and program design, the Pharmac team performs specific special designs for different operating conditions. Without increasing costs, they achieve this through pipeline connection methods, logical sequences, and cleaning strategies. During CIP, they ensure that large pipelines are cleaned at the correct velocity, ensuring effective cleaning, short overall cleaning times, and low cleaning media consumption.
The Pharmac team's special design solutions have been successfully implemented in past projects by the author's team, receiving positive feedback during commissioning and actual use.
CIP design is a critical factor in determining the success of a large biopharmaceutical system. Design considerations need to be multifaceted, and it is important not to be overly fixated on standard designs. The design should be adjusted for each specific operating condition, taking into account the overall layout, actual pipeline conditions, production scheduling, and control methods. Through reasonable early-stage design, it is possible to shorten commissioning times, reduce long-term energy consumption, and lower operational risks, ultimately shortening the time to production and providing better returns and user experiences for the owners.
From Design to Implementation, Pharmac Offers a One-stop Solution For Clean Fluid Process Equipment In the Pharmaceutical Industry.
